Introduction: Iptacopan is a first-in-class, oral, selective inhibitor of factor B, a key component of the complement system alternative pathway. Inhibition of the complement system represents a potential mechanism for treatment of several diseases including paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome. The purpose of this randomized, participant-blinded, placebo-controlled phase 1 study was to assess the effect of supratherapeutic doses on the exposure-response relationship of iptacopan concentration and cardiac conduction, and to assess the cardiac safety and tolerability of ascending, supra-therapeutic single doses of iptacopan.
Methods: Supratherapeutic doses of iptacopan 400, 800, and 1200 mg were included in this study, representing ≤6-fold higher dose than the planned clinical dose of 200 mg. The primary endpoints were change from baseline in Fridericia-corrected QT interval (QTcF) and safety endpoints. The analyses included data from this study pooled with part 1 data (single dose) from the first-in-human (CLNP023X2101) study (n=86). The study population included healthy men and women aged 18-55 years. The relationship between iptacopan plasma concentration and change from baseline in QTcF was estimated using a linear mixed effects model.
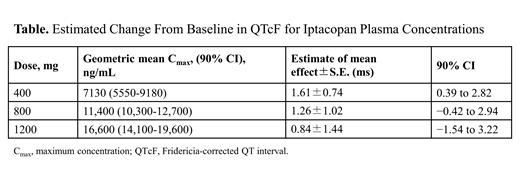
Results: Thirty-two patients were randomized and received placebo (n=8) or iptacopan (n=24). The median age of participants in the 400-mg, 800-mg, 1200-mg, and placebo groups was 51.5, 35, 36, and 37 years, respectively. The upper bound of the 2-sided 90% CI for linear mixed effects model-derived placebo-adjusted change from baseline in QTcF at the geometric mean C max was 2.82 ms for 400-mg, 2.94 ms for 800-mg, and 3.22 ms for 1200-mg doses. Hence, there was no evidence of clinically meaningful QTc prolongation ( Table). The exposure-response analysis of PR and QRS intervals showed no iptacopan concentration-related effects. While the pre-specified and more simple exposure-response analysis of heart rate using change from day 1 baseline (0 hour) revealed an increase of 3 to 5 beats per minute, in a post hoc analysis using 24-hour, time-matched comparison for each participant at each time point before and after study treatment (the more common and gold-standard analysis), iptacopan had no effect on heart rate. No deaths, no moderate or severe treatment-emergent adverse events, and no treatment cohort discontinuations due to adverse events were reported.
Conclusions: Three supratherapeutic doses were well tolerated and were not associated with any relevant change in QTcF. Iptacopan did not affect PR or QRS intervals and a time-matched post hoc analysis showed no effect on heart rate. These results indicate that the anticipated clinical dose of iptacopan (200 mg twice daily) has no adverse clinical effects on cardiac function.
Disclosures
Schmouder:Novartis Pharmaceuticals Corporation: Current Employment, Current holder of stock options in a privately-held company. Kaetterer:Novartis Pharma AG: Current Employment. Kulmatycki:Novartis Institutes of Biomedical Research: Current Employment. Nidamarthy:Novartis Healthcare Pvt Ltd: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal